When the decision was made by BASF to bring a new triazole fungicide to market, an entirely new approach was needed to ensure it passed through the EU’s difficult approvals process. CPM tells the story.
With Revysol we put the cart before the horse.
By Tom Allen-Stevens
They say necessity is the mother of innovation and you could argue it’s never been more necessary to bring new fungicide chemistry to growers. But perhaps the true innovation behind Revysol, the first new azole to reach the UK market for 15 years, has been the approach BASF has taken in the face of the tough EU regulatory regime.
“With Revysol we put the cart before the horse,” notes UK and Ireland head of technical management Rob Gladwin. “The EU’s aversion to conventional crop protection is a major challenge for the industry, growers and eventually for consumers.

Right from the start, research teams looked specifically for chemistry that does not harm mammals, recalls Rob Gladwin.
“For the azoles there is the backdrop of concerns over endocrine disruption, so we addressed this head on – right from the start, our research teams looked not just to target enzymes in the fungal pathogens, but also specifically for chemistry that does not harm mammals.”
But it’s not just Revysol’s regulatory profile that’s of note, according to Dr Martin Semar, senior principal scientist with BASF and one of the first to test it in the field. “To have an active that’s so effective against so many diseases in so many crops, that’s really rare,” he says.
The quest for new chemistry started around eight to ten years ago, Rob recalls. “The decision was made to look for a new azole. The efficacy of current chemistry was shifting and there was great uncertainty over whether the majority of the existing azoles in the market place would get through the very difficult EU approval renewal process. BASF has always understood the need for the precautionary principle, but we feel it’s been abused – to succeed it’s not just about getting the science right, but also the politics.”
So in a new molecule, there were two key features the BASF research teams were tasked with finding – the right selectivity and also a favourable regulatory profile. “The difficulty is that the target enzymes in fungal pathogens are closely related to those found in mammals,” notes Rob.
The breakthrough came with the development by the biochemistry team of new enzyme assays. Dr Ian Craig, a computational chemist, joined the team in 2011. “The assays test the efficacy with which the DMI chemistry, which includes azoles, inhibit the fungal target site, CYP51 – blocking this enzyme prevents a fungal cell from producing ergosterol causing it to die,” he explains.
With the new assays the team could quickly screen a large number of molecules in their quest for those that were highly effective. What was entirely new, however, was the screening programme running alongside this. “In tandem, the team set up similar assays to test the chemistry for its effect on CYP19. This enzyme target site is found in mammals and is associated with unwanted side effects.”
The team screened hundreds of compounds and received very quick feedback, not just on how effective they were, but also how safe. And this is where Ian’s specialism comes in, but what does a computational chemist do?
“I work closely with the chemistry teams and use algorithms to effect different simulations of how a molecule would bind to a target site and inhibit an enzyme,” he explains. “It’s a branch of chemistry that’s been in use for some time, but the technology we employ today is highly advanced. The 3-D models we produce help chemists prioritise and then narrow down the molecules they want to investigate and develop.”
Together, the team discovered a unique property of an entirely new family of triazoles – this molecule has a ‘hook’. “It’s an isopropanol linker. This moiety is a small part of the larger molecule and it’s unique for a DMI. We synthesised it and noticed very strong fungicidal effects.”
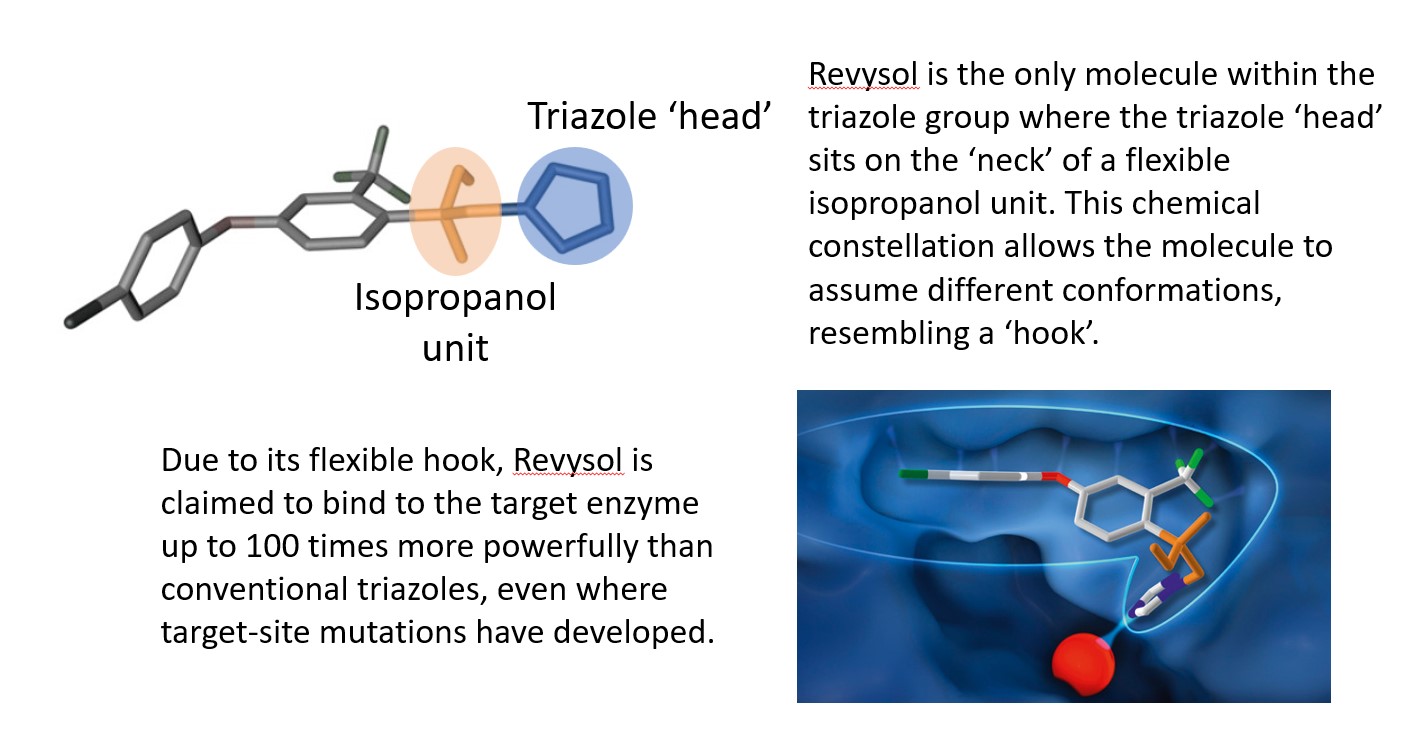 Ian’s simulations showed the ‘hook’ allows the molecule to bind very tightly to the target site. “What’s unique is that it’s flexible – it not only fits perfectly into the CYP51 binding site but it also has the flexibility to adapt if this binding site changes, unlike other azoles. We think that helps with the control of septoria isolates that have evolved mutations,” he notes.
Ian’s simulations showed the ‘hook’ allows the molecule to bind very tightly to the target site. “What’s unique is that it’s flexible – it not only fits perfectly into the CYP51 binding site but it also has the flexibility to adapt if this binding site changes, unlike other azoles. We think that helps with the control of septoria isolates that have evolved mutations,” he notes.
For Ian, both the discovery of this molecule with its unique properties as well as the journey to find it represent the big achievements behind Revysol. “DMIs have a mode of action which is inherently interesting. But the approach we took has taken our work in a new direction and has become the standard way we start the process of new active ingredient development,” he notes.
“We do everything we can to put forward the right molecules into our development pipeline so we don’t have problems later, but we see so many that fail. It makes it all the more rewarding when you find one that succeeds.”
But so far, the new molecule had only got as far as the lab. How would Revysol perform in the field? Martin put it to the test against a broad range of key diseases across globally important commercial crops. “When we saw how highly active it was across such a wide spectrum of situations, we realised this was the one to take forward,” he says.
“It’s not only the high activity on major diseases such as Septoria tritici – it’s not so difficult to find this. It’s also the selectivity – having the ability to use it across a large number of commercial crops in many different countries. What we also noticed was that Revysol was effective on shifted septoria strains. It’s the combination of these factors that made it a potential blockbuster.”
After the first initial and promising results, the team extended the testing program, broadening it to more crops and diseases, first in the greenhouse then on a few research stations and then globally under various conditions.
The regulatory studies of new active substances also continued in parallel with the efficacy work. “The development of Revysol heralded this new focus in our R&D activities, targeted on the regulatory profile, which now start at a much earlier stage and in far greater detail,” explains Martin.
“This is different to the studies we carried out in the past where biological optimisation had the higher priority. All told, it was a long and expensive process, but we built up a complete picture of both Revysol’s biological efficacy and its regulatory profile.”
The final piece of the jigsaw was to make the molecule mobile within the plant and ensure the product’s stability. Dr Christian Sowa is a formulation chemist with BASF. “The first thing you do with a new molecule is to get to know its structure and its solubility profile. That can be a particular challenge with DMIs as they tend to crystallise, so we use an emulsifiable concentrate (EC) to deliver the right results,” he explains.
“But one property we noticed about Revysol is that it has very poor solubility, so there’s a lack of suitable solvents. That leads you towards complex solvent mixtures, which have their drawbacks in terms of how they may interact in the can or in the sprayer. The upside is that once in the leaf, the low solubility helps – Revysol forms an inner leaf reservoir which slowly moves through the leaf, ensuring long-lasting protection.”
Formulation chemistry has moved on apace since the last azole came to market, however. A new aspect in this field again is computational chemistry, which is often brought in to tailor formulations and model different scenarios. “You have to know how it will behave when the product’s scaled up – you don’t want any surprises when it’s already in use by thousands of farmers.”
Revysol’s broad range of uses was a further challenge – it’s available in no fewer than 30 different formulations to cater for the different countries and crops in which it’ll be used. There’s also the interaction with Xemium once formulated as Revystar XE. “Xemium has different properties – it creates crystal depots on the leaf surface, and the crystals can form in the can if you have the wrong formulation,” notes Christian.
Revysol passed through a gradual process of refining, assessing and addressing questions raised. “Some tests take many months until you have a result, which may be a nice outcome, but raise ten more queries. But what we try to ensure is a formulation that is flawless and performs invisibly – success is when the farmer doesn’t even notice it,” he says.
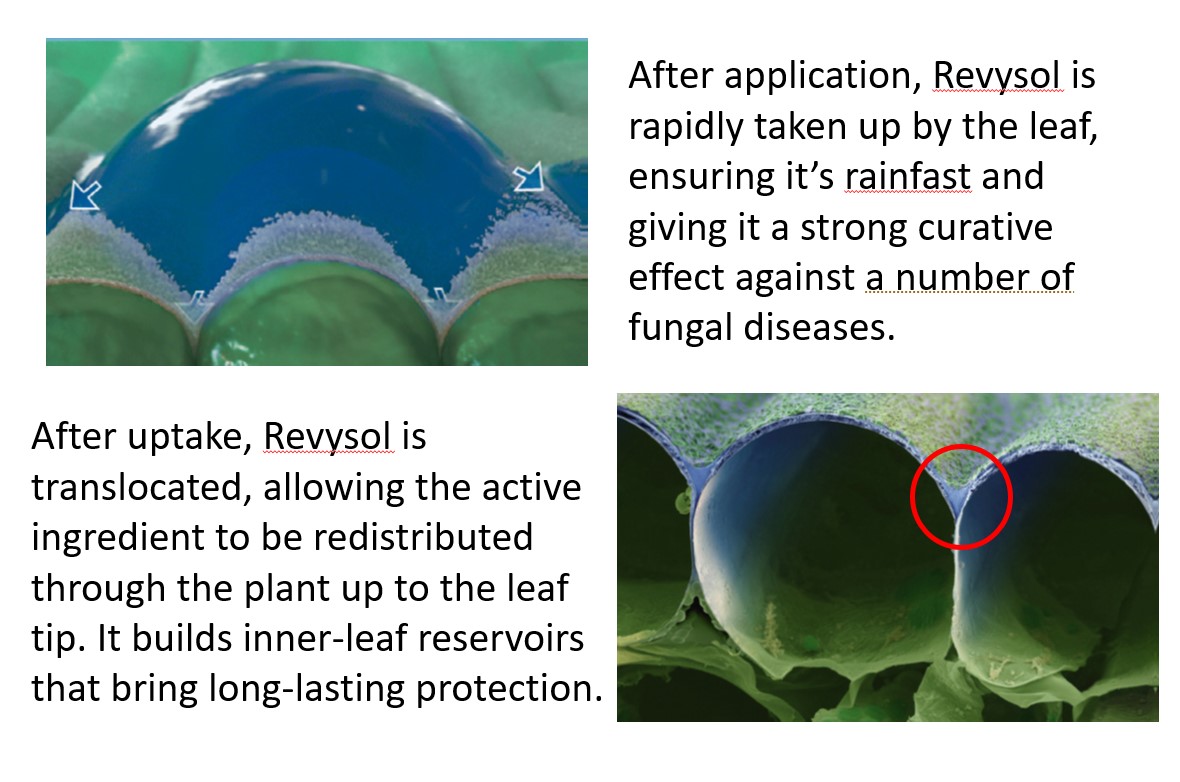 It’s also a key requirement that the delivery to the target is optimised, and it was Dr Marcel Patrik Kienle who ensured this was the case. “What we do is test what happens in the field through lab experiments. We look at how much stays on the leaf, how quickly it’s taken up following application and its movement to the leaf tip,” he explains. “Revysol is barely soluble in water, so not that mobile.”
It’s also a key requirement that the delivery to the target is optimised, and it was Dr Marcel Patrik Kienle who ensured this was the case. “What we do is test what happens in the field through lab experiments. We look at how much stays on the leaf, how quickly it’s taken up following application and its movement to the leaf tip,” he explains. “Revysol is barely soluble in water, so not that mobile.”
So how quickly is it taken up into the leaf? “We found up to 65% of the applied active ingredient got into the leaf within one day, most of this within just six hours. This is important as, once inside the leaf, the active is protected from environmental influences like rainfall or UV-light,” notes Marcel.
“There’s also a remarkably high uptake in cold conditions – 20% is taken up in the first six hours, which compares with a standard formulation where you’d expect nearer 5%.”
Droplets were placed on the leaf between the stem and the leaf tip to assess how quickly Revysol moves through the plant. “You’re looking for a good, even distribution and don’t want it to move too quickly because that can cause phytotoxicity and you get scorch. Equally you’re looking for longevity, so that its strong curative action lasts. We found 10% of the active made its way from the application area into the direction of the leaf tip within a week.”
Numerous small-plot trials and on-farm field-scale evaluations have now demonstrated the properties of Revysol. “But what’s taken the industry by surprise is the speed with which it’s passed the EU regulatory hurdles,” says Rob.
“With Revysol we had the quickest process from submission for active substance approval to registration of the first formulation. BASF developed Revysol by optimising exceptional biological efficacy and an outstanding regulatory profile.”
The registration was also designed to be Brexit proof – applications for Revysol were submitted both through the EU and separately to UK’s Chemical Regulation Directive (CRD), in case the UK had left the EU before approval was granted.
“We’ll be following the same procedure with future product approvals to ensure UK growers always get access to the best chemistry available,” Rob assures. “But approval for Revysol is really good news for global agriculture as well and a stunning endorsement of our R&D and regulatory teams.
“By proactively addressing all of the concerns of the toughest approvals system on the planet, we’ve put a truly innovative product in the hands of growers that will allow them to progress cropping systems around the world.”
Innovation Insight
CPM would like to thank BASF for sponsoring this article and allowing privileged access to staff and materials to help bring it together.




